Background: Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon peripheral T-cell lymphoma most frequently arising around a textured surface breast implant. It has been shown that capsule infiltration (T2, T3 and T4 of the TNM staging system) was an adverse prognostic factor. In this context, chemotherapy (with or without brentuximab vedotin [BV]) may be proposed, considering other factors such as metastatic disease or incomplete resection. Furthermore, the need for adjuvant chemotherapy in patients with stage IIA (T4N0M0) and complete en-bloc excision without residual disease is debated.
Aim: To assess the management of BIA-ALCL with capsule infiltration, in particular: 1) To assess the impact of BV in those patients; 2) To evaluate the need for adjuvant chemotherapy in patients with stage IIA and complete en-bloc excision without residual disease.
Methods: Since 2016, a national multidisciplinary meeting has been implemented by the French National Cancer Institute (NCI) to optimize management of patients with BIA-ALCL, particularly those with advanced disease. Meanwhile, a national registry has been set up by the LYSA, in collaboration with health authorities. Central pathological review has been carried out within the French NCI-labeled Lymphopath network.
Results: From 2009 to 2023, 125 patients with BIA-ALCL were recorded, of which 111 (94 from France and 17 from Belgium) had complete data and were included in the present analysis. Overall, median age at diagnosis of BIA-ALCL was 58 years (24-82 years). Reasons for initial implantation were breast cancer reconstruction (n=54; 49%), cosmetic (n=45; 41%) or other (n=12; 10%). The median interval from first implantation to diagnosis of BIA-ALCL was 12.3 years (4-40 years). The median number of implants in the breast involved by BIA-ALCL was 2 (1-8), including at least one silicone implant in 95 (86%) patients, and at least one textured implant in all informative cases (n=102; Most often Allergan Biocell implants). At diagnosis of BIA-ALCL, 78/110 (71%) patients presented with periprosthetic effusion only, 20 (18%) had effusion and a breast mass, 6 (5.5%) had a breast mass only, and 6 (5.5%) had neither. At pathological examination of the capsule, T-stage was T1 (confined to effusion or a layer on luminal side of capsule) in 73/111 (66%) cases, T2-T3 (capsule infiltration) in 5 (4.5%) cases, T4 (lymphoma infiltrates beyond the capsule) in 32 (29%) cases, and unclassifiable in 1 case. Moreover, 18/111 (16%) patients had at least one non-breast extranodal involvement.
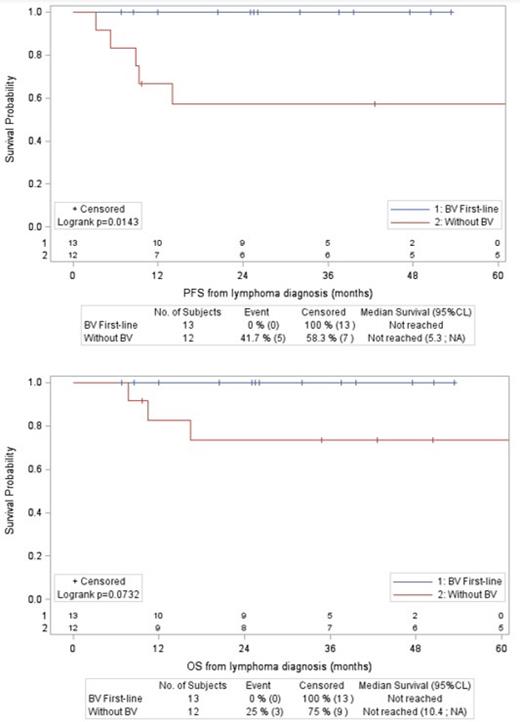
Among the 36 patients with capsule infiltration (5 T2-T3 and 31 T4), 13 (including 11 T4) were treated with BV-CH(E)P regimen, 12 (all T4) with a CHOP/CHOP-like regimen, and 11 (including 8 T4) did not receive any chemotherapy. The main reasons for receiving chemotherapy were metastatic disease, incomplete resection, or no surgery. Patients who did not receive chemotherapy had a localized disease (3 stage IB/IC and 8 stage IIA) with complete en-bloc excision and no residual disease. Patients treated with BV-CH(E)P regimen and CHOP/CHOP-like regimen had similar characteristics without significant differences regarding age, clinical presentation, stage and surgery. After a median follow-up of 47 months from BIA-ALCL diagnosis, for BV-CH(E)P and CHOP/CHOP-like groups, the respective 4-year PFS rates were 100% and 57% (p=0.01), and the respective 4-year OS rates were 100% and 73% (p=0.07). Regarding the 11 patients who did not receive chemotherapy, 4-year PFS and OS were both 75%, which corresponds to a single event being a death without progression occurring more than 3 years after the diagnosis of lymphoma (4-year lymphoma-specific survival was 100%).
Conclusions: 1) In patients with capsule infiltration requiring chemotherapy, BV-CH(E)P regimen was associated with improved PFS and OS compared with CHOP/CHOP-like regimen; 2) Patients with stage IIA and complete en-bloc excision without residual disease had favorable outcome without the need for adjuvant chemotherapy. These results should be confirmed prospectively.
OffLabel Disclosure:
Sibon:Roche: Consultancy; Janssen: Consultancy; AbbVie: Consultancy; Takeda: Consultancy. Schiano De Colella:Janssen, Sanofi, GSK, Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer, Amgen: Other: Accomodation. Oberic:roche: Honoraria; gilead: Honoraria; incyte: Honoraria; BMS: Honoraria; AZD: Honoraria. Laurent:Janssen Pharmaceuticals: Honoraria; F. Hoffmann-La Roche AG: Research Funding. Bachy:Kite, a Gilead Company: Honoraria, Other: Personal Fees; Roche: Consultancy, Honoraria; Amgen: Research Funding; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Novartis: Honoraria, Other: Personal Fees; Incyte: Honoraria; Takeda: Honoraria; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment.
Brentuximab vedotin was used off-label in patients with BIA-ALCL.